Lesson 3: Energy and Matter
OBJECTIVES
Name and describe the three basic forms of energy: potential, kinetic, and electromagnetic.
State and apply the law of conservation of energy
Differentiate between the terms temperature and heat
Compare the Fahrenheit, Celsius, and Kelvin temperature scales
Convert temperatures from Celsius to Kelvin temperature scales
Explain what is meant by absolute zero
Name and describe the four states of matter
Compare physical and chemical properties of matter
Define and recognize examples of elements.
Learn the correct way to write the symbol of an element
Define and recognize examples of compounds
Energy
Energy is the ability to do work to accomplish change. Energy is found in many forms: light, heat, sound, mechanical, electrical. Energy can be classified into three basic forms. Potential energy refers to stored energy. Potential energy can be stored in the form of chemical bonds (chemical potential energy) or as energy of position (e.g., a rock at the top of a hill).
Kinetic energy is the second basic form of energy. Kinetic energy is the energy possessed by an object as a result of its motion (e.g., a baseball traveling through the air towards home plate).
The third major form of energy is electromagnetic energy, also known as radiant energy. This refers to energy traveling in waves, in a straight line, at the speed of light. This type of energy includes the visible light of all colors that we can observe with our own eyes. Electromagnetic energy includes the ultraviolet energy (UV rays) that can give us sunburn. Electromagnetic energy also includes the infrared energy that radiates from a conventional oven, when we open the door to check our food. We do not see infrared energy, but we can definitely feel it on our hands and face.
There are specific patterns of behavior of different forms of energy that have been observed and organized into overarching principles called laws. The Second Law of Thermodynamics states that energy is neither created nor destroyed in ordinary chemical reactions. This law means that the total amount of energy remains the same, but that energy can be transferred from one place to another, or transformed from one form to another. An example of transferring energy is placing a pan of water on the stove top for several minutes. The infrared energy from the burners heats first the pan and then the water – the heat energy is transferred from an area of high amounts of heat energy (the burner) to an area of relatively low amount of heat energy (the water). An example of transforming energy from one form to another is burning fuel in an automobile. The combustion reaction allows the energy stored in the chemical bonds of the fuel, to be released as heat energy during the course of the reaction. Operating a car involves a series of transformations and transfers. For example, burning gas to drive a car involves converting chemical energy to thermal energy to mechanical energy.
Combustion processes are the most common way we have to release chemical energy in a useful form, heat. Heat is defined as the amount of energy in a system. Temperature is a measure of the average kinetic energy of a system (or the average energy of the molecular motions of a system). Basically, each molecule has its own kinetic energy. The average kinetic energy is a measure of the average kinetic energy of all the molecules in a substance. Electrical power plants use a series of transformations to convert coal, oil, or water power (hydropower) into electricity. The energy is stored as potential chemical energy in the coal and oil and, for hydropower, as energy of position in the water at the top of a fall.
Photosynthesis and Energy Storage
Energy can also be converted from its electromagnetic form into storage. Photosynthesis is an example of this type of storage process. Ultraviolet light from the sun provides the energy plants need to convert carbon dioxide (CO2) and water (H2O) into glucose (C6H12O6), which the plant can use for energy, or as a building material for plant structures. The second product of this reaction is breathable oxygen gas, a real bonus for us!
Energy + CO2 + H2O ® C6H12O6 + O2
Sunlight energy is really not just the light we see, but also the infrared heat energy we feel beating down on our skin, the ultraviolet rays that can tan or burn and other electromagnetic (radiant) energy. Fossil fuels such as coal, petroleum (oil), and natural gas all originate in photosynthesis. These fuels begin as plant and animal materials composed of the elements carbon (C), hydrogen (H), and oxygen (O). After millions of years of geological changes in the earth’s crust, these materials would be buried by tons of rock and dirt. Under the rock and dirt, sunlight and oxygen from the atmosphere were not available. Over time, the extreme conditions of the rock pressing down on the materials, combined with the extreme heat from the earth’s core, caused the plant and animal materials to react, releasing water and oxygen. Thus, the fuels that we know today, coal, petroleum, natural gas, all contain carbon and hydrogen. These are known as fossil fuels because they are generated from fossils. Some molecules found in crude petroleum also contain a small proportion of oxygen, but it is very small compared to the amount of carbon and hydrogen. The high relative percentage of carbon and hydrogen in substances we typically regard as fuels, is one of their defining characteristics.
The processes by which fossil fuels are formed present a problem. These processes occur so slowly that the amount of fuel that any person requires during their lifetime cannot be regenerated during that lifetime. In fact, many highly industrialized nations such as the United States, Germany, Great Britain, are currently using fuels many times more quickly than they can be regenerated. You may even be aware of the newspaper articles and reports that reveal the serious concern many people have over the amount of fossil fuels that are available to us. Since fossil fuels cannot be regenerated by natural processes on a useful human time scale, we can not count on more of these fuels than we already have. Therefore, these resources are considered nonrenewable. A resource that cannot be replenished will eventually run out.
">That is the concern many people have about our rapid rate of use of fossil fuels. Some projections indicate we may run out by the year 2050. Some estimates indicate that if we conserve resources we may be able to extend out supply for another 100 years or more. In any event, there is still a strong possibility that we may have to turn to other sources of energy in the future. Many countries and many private groups are already exploring the development of other energy sources (called alternate energy sources). We will discuss some these in a later chapter.
Another concern associated with fossil fuels involves the products of the combustion reactions that release the stored energy in fuels. For example, the combustion of methane (CH4, the hydrocarbon fuel we know as natural gas) in air produces carbon dioxide and water according to this balanced equation:
CH4 + 2 O2 ® CO2 + 2 H2O + Energy
Carbon dioxide is one of the major greenhouse gases. A greenhouse gas is a substance containing two or more elements that absorbs infrared energy inside the earth’s atmosphere and radiates that energy back to earth. The gases in our atmosphere behave like the walls of a greenhouse, trapping in heat to keep the temperature suitable for supporting plant and animal life. It is very beneficial that our atmosphere functions as a greenhouse. Without an atmosphere, the earth would be so cold that we could not survive.
The problem with greenhouse gases such as CO2, and combustion reactions that we use to release energy, is that we are producing CO2 more rapidly than the plants on earth can use it in photo-synthesis. There are also other processes besides photosynthesis that use CO2. Even combining all the CO2 used up by natural processes on earth, human activities are still contributing to a rapid increase in the amount of CO2 in our atmosphere. More CO2 could mean more energy trapped inside the atmosphere. More energy could mean that the earth would warm up enough to begin melting the polar ice caps and causing drastic changes in local climates all over the world. There is much discussion over the controversy of what exactly will happen in the future, and to what extent human activities can affect the composition of our atmosphere.
You may want to examine more information to come to your own conclusions. Learning to examine the available information to draw your own conclusions is an important skill, but it takes practice!
Converting Temperatures: Absolute Zero and the Kelvin Scale
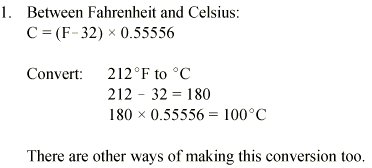

The absolute, or Kelvin, temperature scale is often used in chemistry, particularly when working with gas laws. The Kelvin degree is the same size as the Celsius degree. Unlike the Fahrenheit and Celsius temperature scales, which are based on the boiling and freezing points of water, the Kelvin scale is based on absolute zero (0 K). Absolute zero is equivalent to -273.15°C and is the lowest possible temperature. It is the point where all molecular motion ceases, and where an ideal gas has a volume of zero. To convert from a temperature from Celsius to Kelvin, just add 273.15 to the Celsius temperature.
What Is Matter?
Matter can be defined as anything that has mass and takes up space. That means everything! Keep in mind that atoms are the smallest bits of matter that retain a complete set of physical and chemical properties. Each atom has a specific arrangement of subatomic particles, which we will discuss in Chapter 4. We can identify which atoms are alike and which are different, by observing their properties, and by observing their structure. Atoms are then organized into categories, by structure. The categories are called elements. Keep in mind that all atoms of an element have a complete set of physical and chemical properties that are characteristic of the element. You may have heard about interesting newly discovered particles that are the result of breaking apart atoms or parts of atoms in high-energy collisions. While this is interesting to know, keep in mind that almost all of the changes we observe everyday, on an atomic (micro) level, or on a larger, bulk (macro) level, can be accounted for by the properties of different atoms, which we can identify as specific elements.
Now, on to physical and chemical properties! Physical properties can be observed without changing the composition of a substance. Examples of physical properties are shape, color, and state of matter. We will examine states of matter very soon. Chemical properties can only be observed when you are changing the identity of a substance, through a chemical reaction. Reactivity is a chemical property, referring to how quickly and easily something reacts. For instance, not all materials are combustible (able to be burned as fuel). Combustion or burning reactions are common, and are familiar to us. Our cars use internal combustion engines and burn gasoline as fuel. When we cook out on a grill, we use charcoal as fuel.
States of matter refer to the physical arrangements of substances. There are three common states of matter: solid, liquid, gas. A fourth state of matter, plasma, is also known. Each state has specific characteristics associated with it. We will describe the characteristics of solids, liquids and gases, by considering a sample of a common substance, water, as our example in the following table:
|
CHARACTERISTICS OF STATES OF MATTER |
|||
|
Solid |
Liquid |
Gas |
Plasma |
|
Definite Shape |
No Definite Shape |
No Definite Shape |
No Definite Shape |
|
Definite Volume |
Definite Volume |
No Definite Volume |
No Definite Volume |
|
|
|
|
High Energy Particles |
|
Example |
Example |
Example |
Example |
|
ice (solid water) |
water in the drinkable form with which we are most familiar |
steam |
the sun |
Plasma is different from the other three states of matter. Plasma is a high-energy state that is not necessarily composed of one specific substance. Plasma is not commonly found on the surface of the earth. We can, however, generate small amounts of plasma in scientific instruments under controlled conditions.
Let’s examine our three common states of matter in more detail. Solids have a definite shape and volume. An ice cube holds it shape, and only takes up a certain amount of space. As the ice melts, we see that the liquid that forms, water, no longer has a specific shape. The liquid water takes the shape of the container it is in, or spreads out in an indefinite shape it is on a surface such as a counter top. The water in an ice cube will only spread so far; it does have a definite volume. You cannot wet the entire kitchen floor with one ice cube!
When we boil liquid water in a pan on the stovetop, we observe a cloud above the pan. The liquid water has evaporated into gaseous water, steam. We can cool the gas cloud, and it will condense, or return to the liquid state. You have seen water condense, if you have ever boiled water and noticed that the countertop nearby (which is cooler) has water drops on it. If you have a cool beverage in a glass, and water appears on the outside, your cup is not leaking, you have simply condensed water from the air on the cool surface of the glass.
To melt the ice, we do nothing more than remove it from the freezer. The warmth of the air provides sufficient energy to melt the ice to liquid. But to boil water, we need a source of energy, and a lot of it! Our stovetop burners or microwave oven can provide the energy.
To sum up what we know about the physical states of matter: the identity of the substance, water, does not change just because the ice melts or the water boils. The water is still water.
We can relate what we know about states of matter (a physical property) to the larger topic of physical and chemical properties. Physical and chemical properties can be remembered best, when we use examples of physical and chemical changes to remind us.
Ex 1. Physical changes
|
Chopping wood |
Boiling water |
Alcohol evaporating |
|
Butter melting |
glass breaking |
Wire bending |
Physical changes are often reversible, and do not change the identity of the substance. Chopped wood is still wood.
Ex 2. Chemical Changes
|
Boiling an egg |
Iron rusting |
|
Wood rotting |
Milk spoiling |
Boiling an egg is not easily reversible. In fact, boiling (cooking) an egg causes a chemical change in the substances in the egg, making cooked eggs more digestible for humans. Rusted iron is not good for building anymore; burned wood cannot be used as fuel again.
Elements and Compounds
Elements are organized on the Periodic Table by atomic structure, which we will discuss in more detail later. The Periodic Table also reveals the periodic law, which states that the physical and chemical properties of the elements are consequences of their atomic numbers. Elements are organized into groups (vertical columns) with similar physical and chemical properties. All atoms of the same element have the same complete set of physical and chemical properties belonging to that element. There are about 110 known elements. Many Periodic Tables do not show 110 elements, if they were printed or published before all the elements were discovered. The first 40 elements are encountered most often. Many of the newly discovered elements are only known to exist under laboratory conditions. Elements are identified by a name and a short version of the name, or symbol. The symbol may be one, two or three letters. Each element has a unique name and symbol. Carbon has symbol C, oxygen, O, calcium, Ca. The first letter of a symbol is always capitalized. The element symbol can represent one atom of the element or a group of atoms, depending on the context. Elements are classified as pure substances, because each element has a unique set of physical and chemical properties.
A molecule is a group of two or more atoms that are chemically combined. The atoms can be the same, as in O2, a molecule of oxygen gas. The atoms can also be of different elements; a substance that contains two or more elements in fixed proportions is called a compound. Water (H2O), carbon dioxide (CO2), and table salt (sodium chloride, NaCl) are all compounds. The law of definite proportions states that elements in a pure substance will always exist in the same proportions. Water is always two hydrogen atoms and one oxygen atom per molecule of water, H2O. Measured by mass, water is 11.1% H and 88.9% O. Hydrogen peroxide has the formula H2O2, which is 5.9% H and 94.1% O. Water, H2O, tastes good to drink and we must have it to live. Hydrogen peroxide, H2O2, is a disinfectant, used to kill germs in cuts and scrapes. Do not drink hydrogen peroxide!
DISCUSSION: A New Language
Here you start to look at the letters and words that chemists use to talk about chemistry. If you think it looks like a different language, you are correct. There are specific, correct ways of expressing yourself in chemistry.
We start here with the basic letters of the language: the symbols for the elements. The chemist puts these symbols together into words and the words into sentences. A big part of learning chemistry is learning this language.
The symbols for elements are written with the first letter capitalized and the remaining letters of the symbol in lowercase. Why is this important?
Co is the symbol for cobalt, a metallic compound like iron with a slightly pink color in it.
CO is carbon monoxide, a colorless gas which attaches to your hemoglobin and prevents the passage of oxygen to your cells.
Notice that if you write the symbol for cobalt incorrectly with both letters capitalized, you would be saying something very different.
LAB HINTS
Some elements are attracted to magnets; check around the house, particularly on the refrigerator! An element attracted to a magnet could be separated from the other substances in the group. Some substances are soluble in hot water, but not in cold. Try cold water to dissolve one substance, and set the water aside. Try a separate sample of warm water (handle with care!) to try to dissolve something else from the mixture. Allow the water to evaporate from the containers, to observe any solid that remains. Some solids do not dissolve in water. A filter or screen might be used to remove them. Coffee filters and strainers allow some substances to go through, while trapping others.